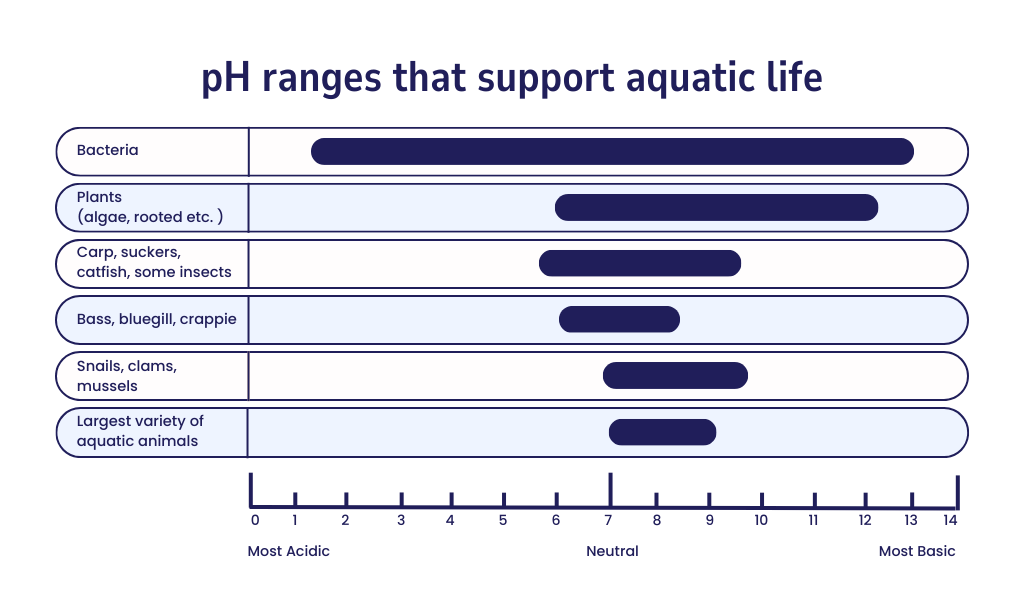
The pH measurement shows how acidic or basic a water body is. The amount of hydrogen ion activity in water determines the level of pH on a scale from 0–14. The lower the pH value, the more acidic the water is. The pH range for natural bodies of water in the United States is around 6.5–9.

The pH of a waterbody is important because aquatic organisms depend on a specific pH range to survive.
The pH is an important water quality parameter. Aquatic animals and plants are adapted to a certain pH range with most preferring between 6.5−8.0. An increase or decrease in pH outside a water body’s normal range can be detrimental to organisms, depending on their sensitivity.
A variety of natural and human factors can influence the pH level of a body of water.
Limno Loan
c/o Illinois-Indiana Sea Grant
Purdue University
195 Marsteller Street
West Lafayette, IN 47907-2033
Phone: 765-496-6009
Email: iisg@purdue.edu
This program is administered by Illinois-Indiana Sea Grant and funded by the Great Lakes Restoration Initiative via cooperative agreement between the U.S. EPA Great Lakes National Program Office and the National Oceanic and Atmospheric Administration.
© 2023 Illinois-Indiana Sea Grant.
All Rights Reserved.
Illinois-Indiana Sea Grant strives to ensure equal access and an inclusive environment.
Designed by Studio 2D