Oxygen is a natural element needed by many forms of life, including aquatic organisms. Most aquatic animals use oxygen dissolved in water for respiration. Oxygen primarily enters water through diffusion from the surrounding air, which can be enhanced by turbulence, and from photosynthesis by aquatic plants.
Dissolved oxygen is measured in units of milligrams/liter (mg/L) or as a percent of saturation (%).

Turbulence at the water’s surface can increase the amount of oxygen that is dissolved into the water from the atmosphere.
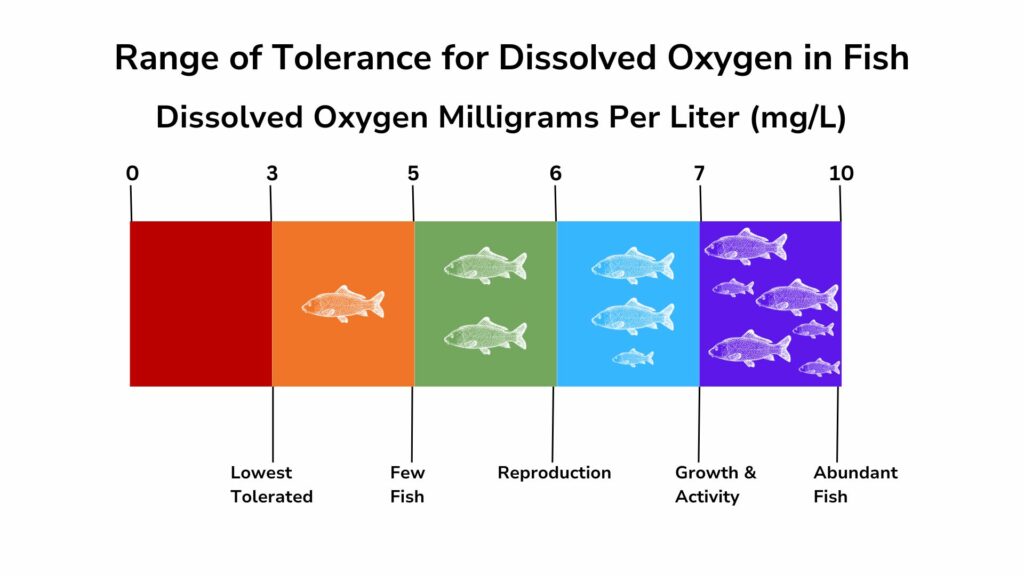
Oxygen is necessary for living things and for many of the chemical processes that take place in water. The amount of dissolved oxygen needed by an aquatic organism depends on a variety of factors, including the species, the organism’s metabolic rate and overall health, and water temperature. Organisms typically have an optimum range in which they do best.
The temperature and salinity of water influence how much oxygen it can hold. Warm water holds less dissolved oxygen than cold water because the molecules are moving faster than in cold water, thereby allowing oxygen to escape from the water. Freshwater can hold more dissolved oxygen than saltwater because saltwater has less space for oxygen molecules due to the sodium and chloride ions it contains. Therefore, the warmer and saltier the water, the less dissolved oxygen it will contain.
As mentioned previously, oxygen is added to water at the surface where gases in the atmosphere come into contact with it. Therefore, the movement of water from wind and waves also can help oxygenate water. In addition, deeper water gets oxygen from the upper layers when mixing occurs.
Dissolved oxygen can also be influenced by humans. For instance, additional nutrients can enter a waterbody in runoff from lawns or farm fields, and cause a large increase in aquatic plant growth. While initially this may raise oxygen levels through photosynthesis, excess algal growth will consume oxygen on cloudy days and at night via respiration. Also, as all the plants die off, oxygen will be consumed by bacteria in the decomposition process.
Limno Loan
c/o Illinois-Indiana Sea Grant
Purdue University
195 Marsteller Street
West Lafayette, IN 47907-2033
Phone: 765-496-6009
Email: iisg@purdue.edu
This program is administered by Illinois-Indiana Sea Grant and funded by the Great Lakes Restoration Initiative via cooperative agreement between the U.S. EPA Great Lakes National Program Office and the National Oceanic and Atmospheric Administration.
© 2023 Illinois-Indiana Sea Grant.
All Rights Reserved.
Illinois-Indiana Sea Grant strives to ensure equal access and an inclusive environment.
Designed by Studio 2D